
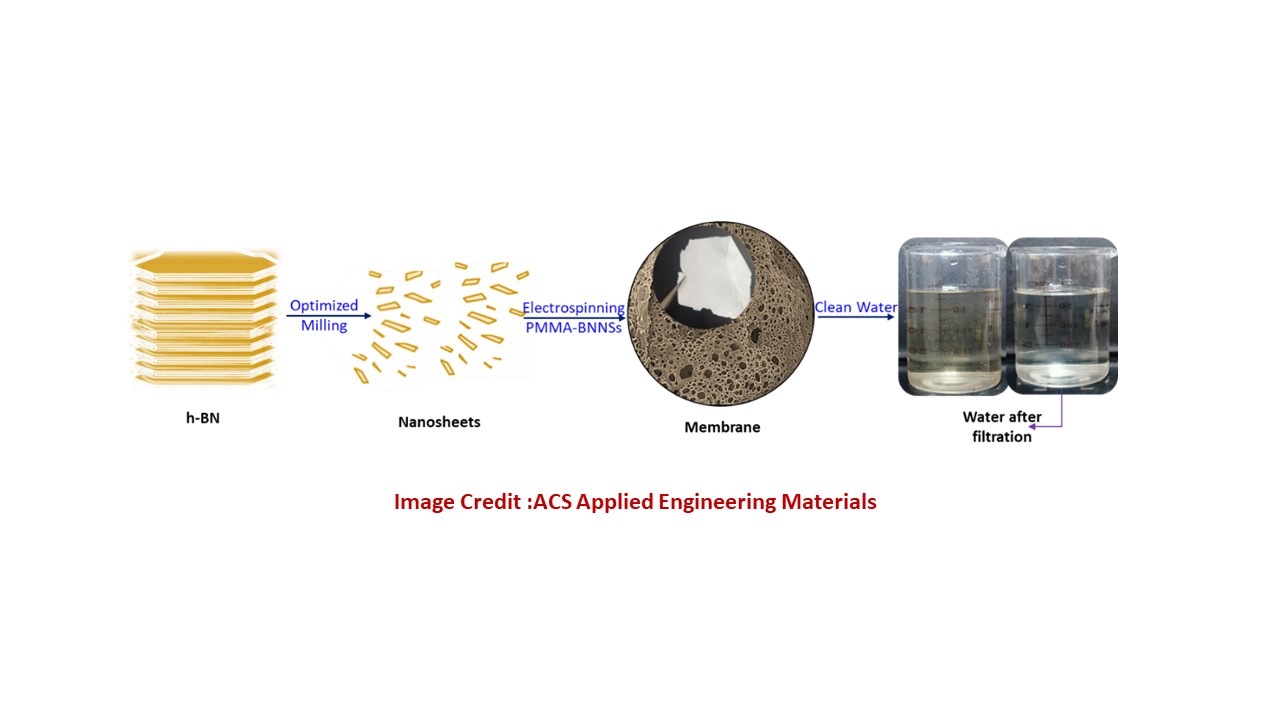
Clean drinking water is becoming increasingly difficult to secure due to pollution, industrial discharge, and the presence of harmful chemicals. Modern water-treatment technologies rely heavily on membranes thin filter-like materials that remove contaminants. However, many commonly used membranes either clog quickly, have limited durability, or cannot efficiently filter very small pollutants. To address these challenges, researchers are now exploring advanced nanomaterials that can make stronger, more efficient, and more long-lasting membranes.
This study focuses (https://doi.org/10.1021/acsaenm.5c00525) on a promising material called hexagonal boron nitride (h-BN). The h-BN is sometimes referred to as “White Graphene” because it has a similar layered structure. Each layer is extremely thin only a few atoms thick, yet strong, chemically stable, and resistant to heat. By peeling or “exfoliating” h-BN into few-layer nanosheets, the researchers produced tiny, ultra-thin flakes with exceptional properties. These nanosheets were then incorporated into a widely used membrane material known as PMMA (polymethyl methacrylate). The result is a new nanoporous membrane ( less then 100 nm), meaning it contains tiny, uniformly distributed pores ideal for filtering pollutants, salts, dyes, and organic molecules from water. The key innovation in this work is the mechanical exfoliation process, which avoids harsh chemicals and produces high-quality h-BN nanosheets with clean surfaces. When mixed into PMMA, these nanosheets improve the membrane properties in several ways:
Overall, the research demonstrates a simple, environmentally friendly method to create advanced membranes using a combination of PMMA and few-layer h-BN nanosheets. These membranes show strong potential for low-cost, high-performance water purification, especially in areas facing water scarcity or industrial pollution. This approach may pave the way for next-generation filtration technologies that are both sustainable and highly effective.
Marc Monthioux November 30, 2025
Hello Santosh
One question : It is understandable how such a porous membrane can act as a screen and retain solid nano-to micro-particles. But how does it work to retain chemical pollutants (molecules, then) since BN layers are electrically neutral, showing no polarity of any kind? Is it grafted with functional groups? Correspondingly, would not the same material using genuine graphene instead of BN be more efficient, since graphene shows some polarity, by the existence of pi electrons?
And one remark: the way a sentence is written, it looks like a single-layer of BN is a few-atom thick, whereas it is one-atom thick.

Rechargeable batteries are essential to modern society, quietly enabling...
View now
Quantum computers represent one of the most profound shifts in the histo...
View now
Graphene: The Strongest Material Ever Discovered...
View now